lateral flow assay covid
It is the most popular format of point-of-care test POCT and quickest and easiest way to detect a targeted molecule. Build your own Human Luminex Discovery Assay with our Luminex Assay Customization Tool.
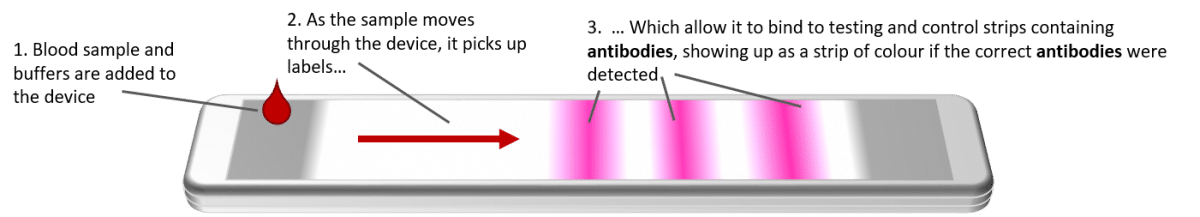
What Tests Could Potentially Be Used For The Screening Diagnosis And Monitoring Of Covid 19 And What Are Their Advantages And Disadvantages The Centre For Evidence Based Medicine
Real-time PCR assay is then used to detect the presence of the viral RNA genome.
. However lateral flow technology has been existence long before COVID. Randox the UKs largest diagnostics company today announces that it is launching the Randox CertiFly App a mobile application designed to securely process and certify results for both pre departure and Day 2 lateral flow tests the latter a. Amid the COVID-19 crisis the global market for Lateral Flow Assays estimated at US78 Billion in the year 2020 is projected to reach a revised size of US112 Billion by 2026 growing at a CAGR.
Should be treated as likely negative and confirmed with a molecular assay if necessary. This lateral flow assay is designed. The usability of self-testing by an individual aged under 18 years has not been determined.
The COVID-19 pandemic has presented an ideal growth opportunity for lateral flow assays due to robust demand for lateral flow assay-based screening tests. Lateral flow or Enzyme-linked immunosorbent assay. Cited in 291 publications.
We validated our method using contrived reference samples and clinical samples from patients in the United States including 36 patients with COVID-19 infection and 42. Whereas other testing methods are based on the detection of antibodies which are only produced as a response to the infection detection of viral RNA allows for the identification of an infection at a. A commercialised molecular assay was used as the reference method and the results showed that the Healgen lateral flow test has high overall accuracy.
The Flowflex SARS-CoV-2 Rapid Antigen Test is the first in this family of lateral flow testing products. We take projects from initial concept through to routine and large-scale manufacturing. Is an RD-focused Chinese biotechnology company that develops manufactures and supplies high-quality lateral flow in-vitro diagnostic IVD rapid test kits as well as revolutionary customized reagent kits to all parts of the world.
It is suggested that individual under 18 years of age should be tested by an adult. The coronavirus antigen rapid test kit is a lateral flow assay that qualitatively detects the presence of nucleocapsid N protein in upper respiratory samples nasal swabs. JOYSBIO Tianjin Biotechnology Co Ltd.
Lateral flow tests have become synonymous with COVID-19 SARS-CoV-2. Our Luminex multiplexing immunoassays include cytokines and chemokines and are tested for sensitivity intra-assay precision inter-assay precision and to ensure assay linearity for validated sample types. 957 Negative Percentage Agreement NPA.
Day 2 lateral flow tests must be taken as soon as possible or at the latest before the end of the second day of arrival by fully. Positive Percentage Agreement PPA. The testing method for 2019-nCoV Coronavirus Covid-19 is a molecular assay based on the detection of the nucleic material RNA within the virus.
This tried-and-true immunoblot assay tests for 30 allergens on one strip. Results are then released to our secure online portal to be downloaded. Instead of sending patient samples to the laboratory the usage of lateral flow test kits can be convenient as they are smaller portable.
COVID-19 Antigen Rapid Test Principle. Kits Reagents one of the segments analyzed in the report is projected to grow at a 61 CAGR to reach US96 Billion by the end of the analysis period. Negative results are presumptive and confirmation with a molecular assay if necessary for.
The Flowflex COVID-19 Antigen Home Test is a lateral flow chromatographic immunoassay. 18 th October 2021. In the combat against COVID-19 pandemic hundreds of POCTs have been developed and are.
We report development of a rapid lateral flow assay for detection of SARS-CoV-2 from respiratory swab RNA extracts. JOYSBIO Tianjin Biotechnology Co Ltd. The COVID-19 pandemic had a positive impact on the market.
Convenient affordable Fit to Fly lateral flow. PCR assay is affected by ethanol contained in a sample thus consuming alcohol just before sampling could be risky and there exists a report of a COVID-19 case with a false-negative PCR assay presenting a past medical history of alcohol use disorder but it remains unknown if alcohol was consumed before sampling 5057. SGTi-flex COVID-19 IgG 09032020.
Vibrant COVID-19 Ab Assay 06042020. COVID-19 Lateral Flow Test. Randox launch authentication app for Day 2 Lateral Flow tests for vaccinated international arrivals.
IgM and IgG CLIA. Nucleic Acid Extraction System and PCR quantitative test kits for HBV HCV HPV 1618 HPV Genotyping. Therefore different industry sectors and different countries use varying terminology to describe what lateral flow tests are.
Rapid Lateral Flow Test LFT has been broadly utilized in detection or diagnosis of numerous disease-related antigens and antibodies. COVID-19 Testing Order your home testing kit now. We also offer flexible solutions for card and strip manufacture through to producing assembled devices and kits.
Lateral flow test LFT Lateral flow device LFD. IgG Lateral Flow Fingerstick Whole Blood. Abingdon Health is a world leading developer and manufacturer of high-quality rapid lateral flow tests across all industry sectors including healthcare and COVID-19.
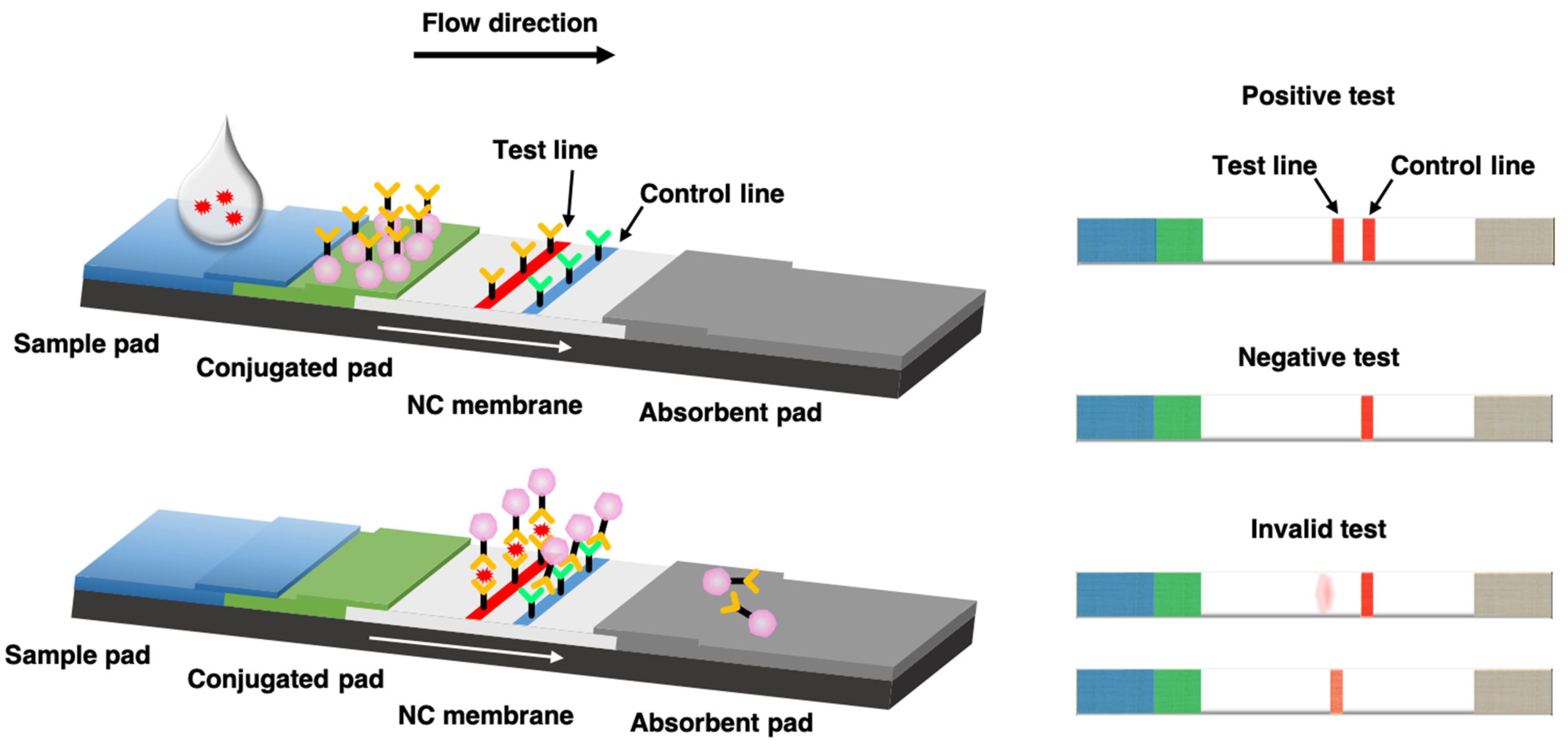
Biosensors Free Full Text Recent Advances In Novel Lateral Flow Technologies For Detection Of Covid 19 Html

Lateral Flow Assay Labels And Conjugation Technologies
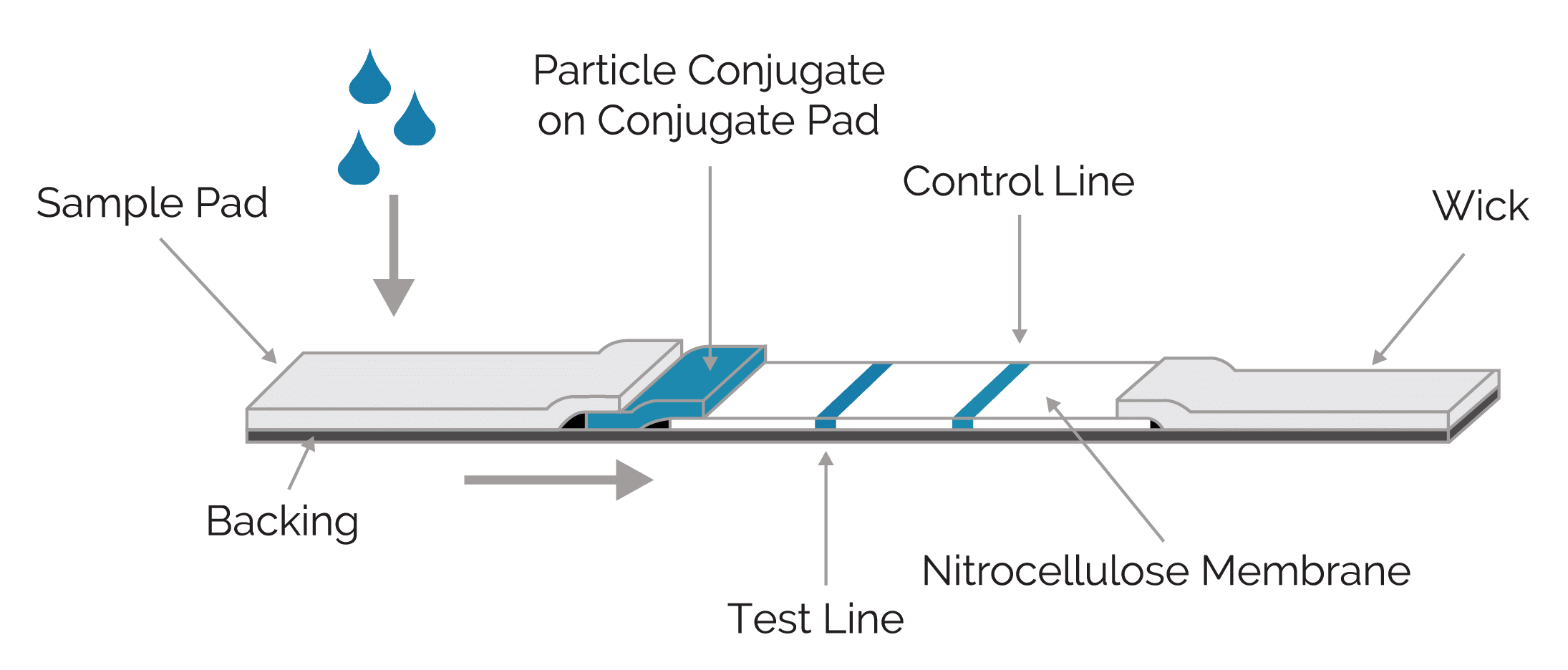
Lateral Flow Assays How Does Lateral Flow Work Dcn Dx

Coronavirus Covid 19 Sars Cov 2 2019 Ncov Assay Kits Lateral Flow Immunochromatography Products Mybiosource
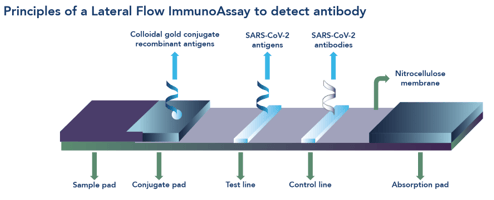
The Rise Of The Lateral Flow Test Everything You Need To Know About Lateral Flow Tests In Five Steps Una Health
Lateral Flow Assay Private Label Joysbio Biotechnology
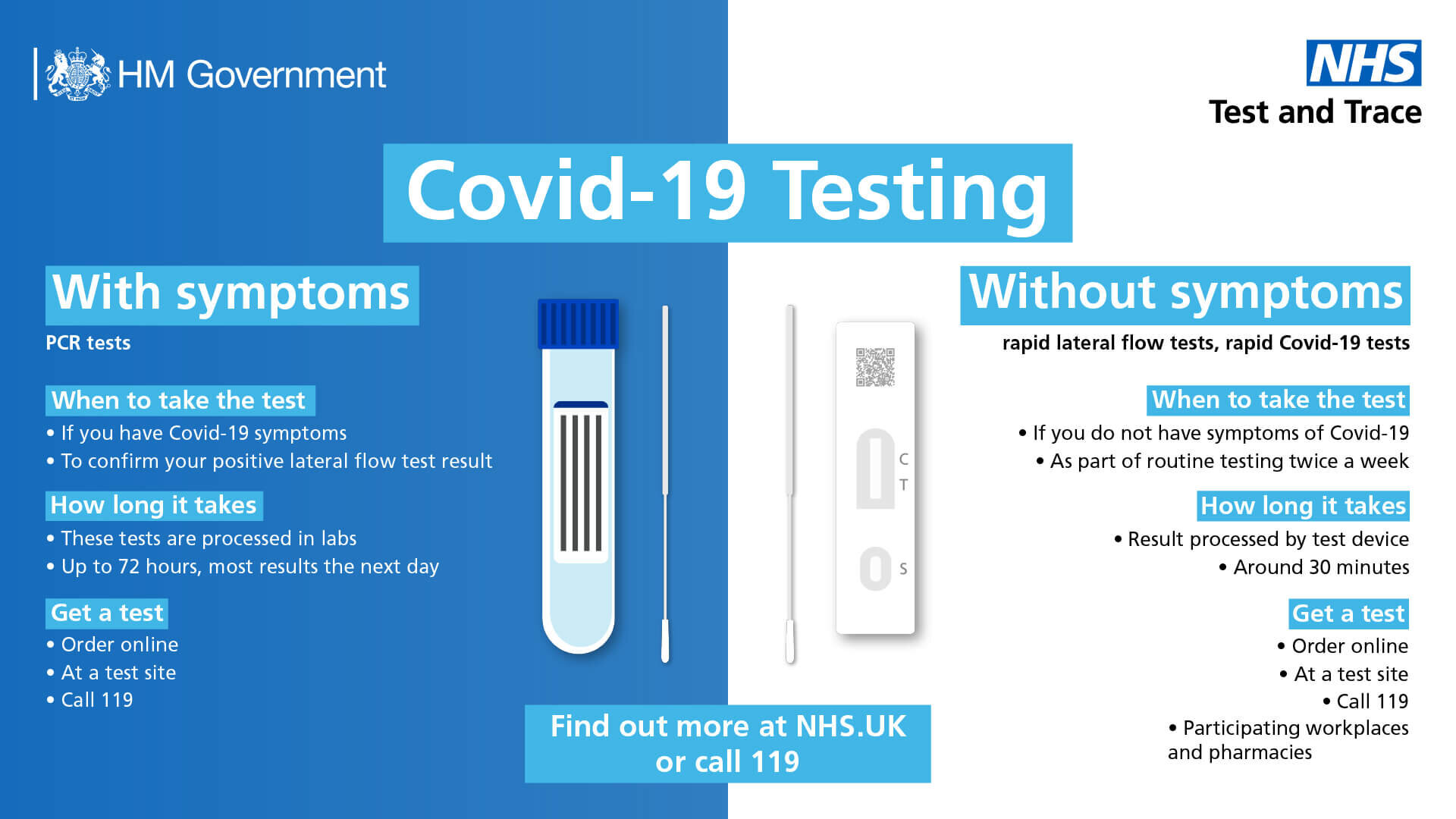
Covid 19 Rapid Lateral Flow Testing A24 Group

Public Health Agency What S The Difference Between A Pcr And Lateral Flow Test Which Test Do I Need And When Find Out More About Covid 19 Tests At Www Pha Site Cvtesting Facebook

How Useful Is Covid 19 Antibody Testing A Current Assessment For Oncologists Clinical Oncology

Developing Diagnostics For Covid 19 Nuffield Department Of Clinical Neurosciences
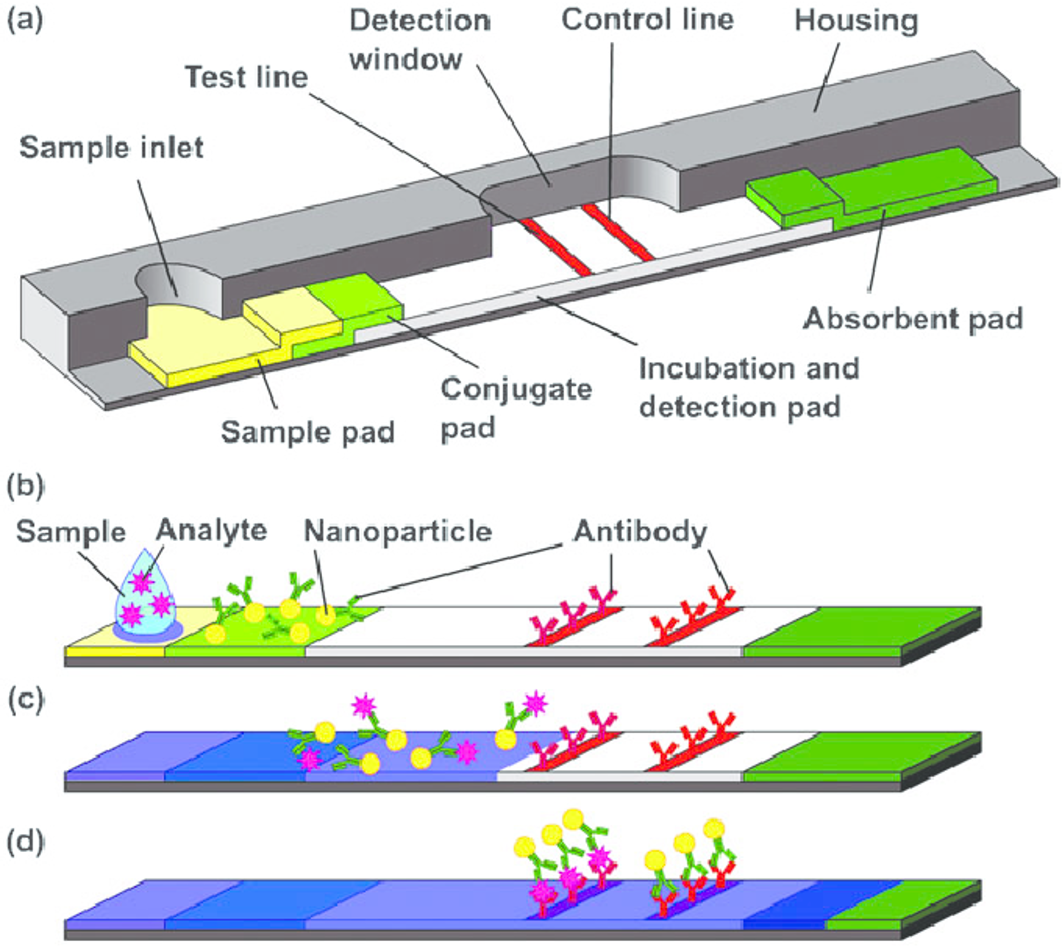
Covid 19 Antibody Test Kits Will Be Available Soon
![]()
Using A Lateral Flow Device Lfd To Test For Covid 19 Key Points To The Correct Technique On Vimeo
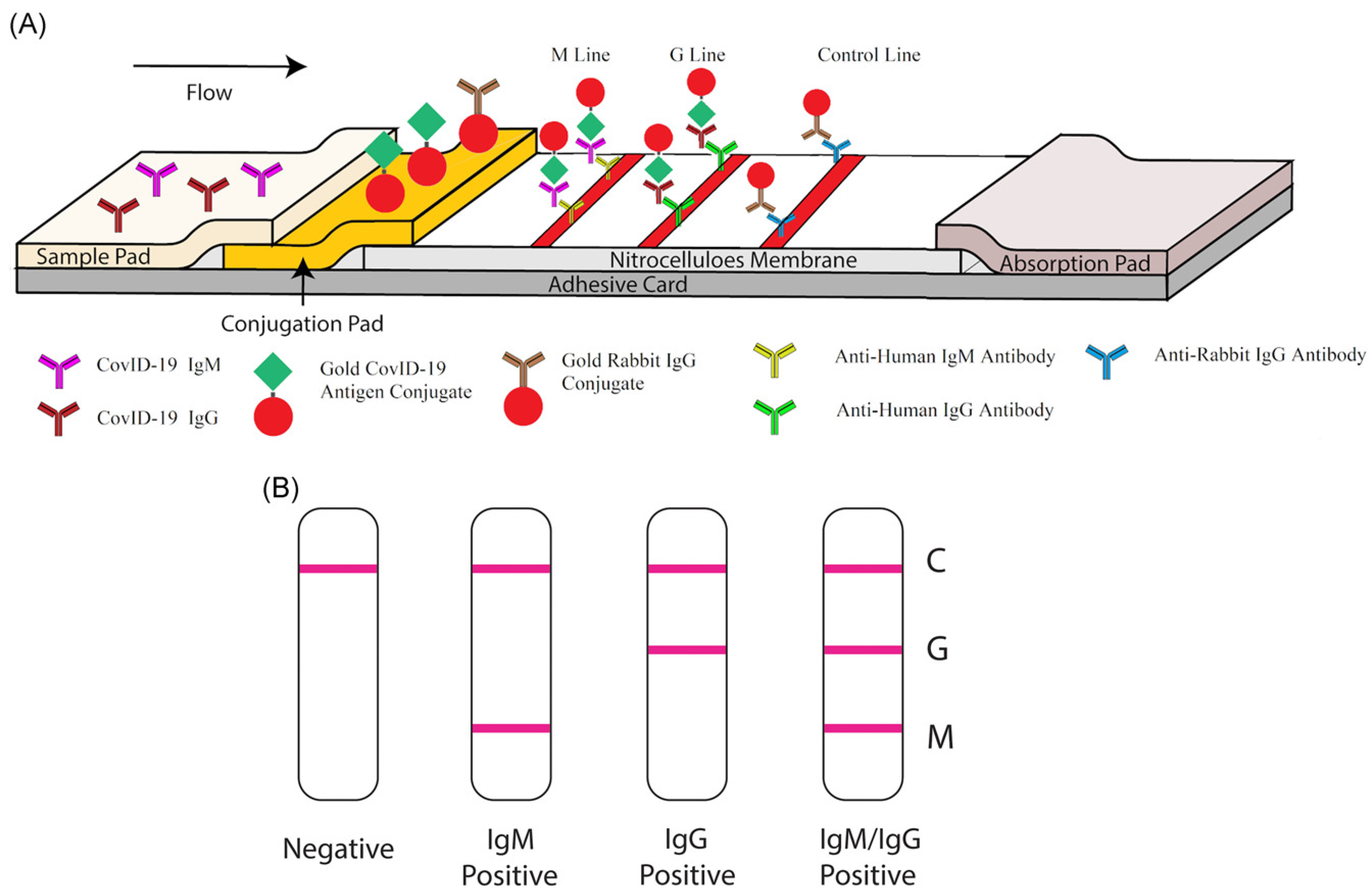
Biosensors Free Full Text Recent Advances In Novel Lateral Flow Technologies For Detection Of Covid 19 Html
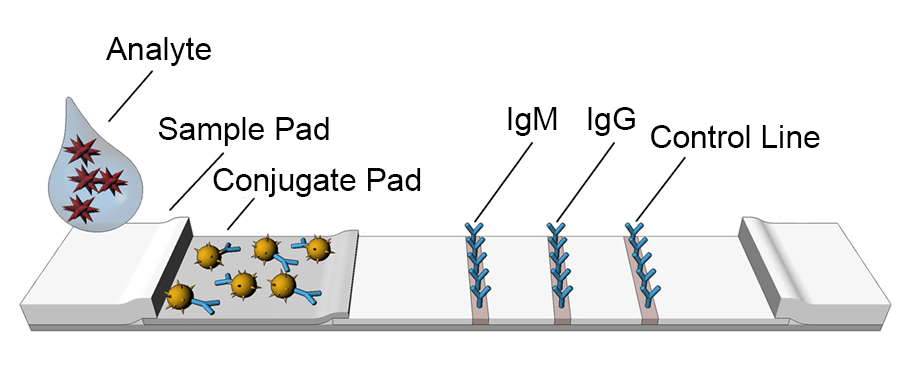
What Is A Lateral Flow Assay And How Does It Work Joysbio Biotech
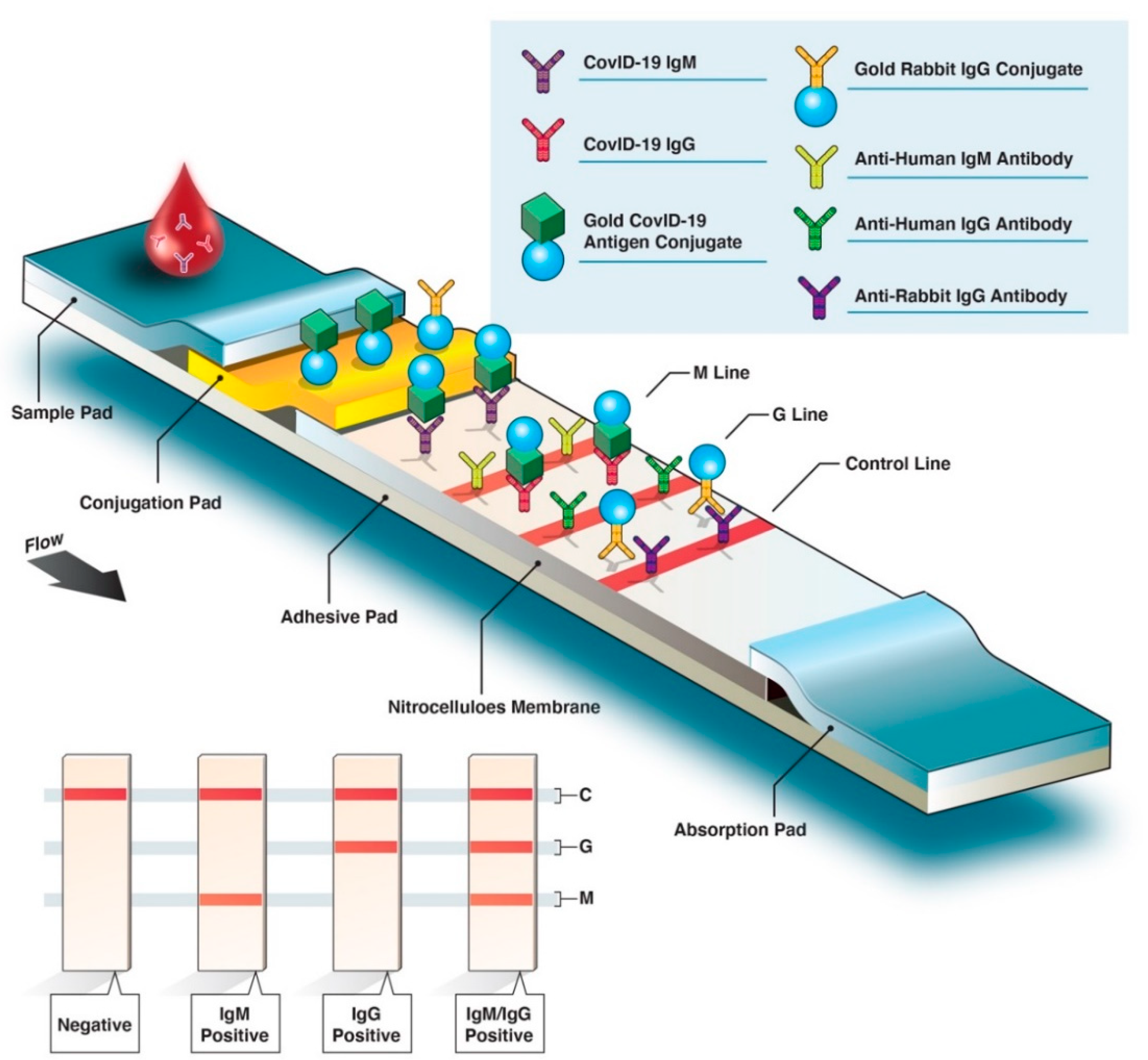
Diagnostics Free Full Text Covid 19 Serological Tests How Well Do They Actually Perform Html

A Point Of Care Lateral Flow Assay For Neutralising Antibodies Against Sars Cov 2 Ebiomedicine

A Point Of Care Lateral Flow Assay For Neutralising Antibodies Against Sars Cov 2 Ebiomedicine

Covid 19 Lateral Flow Self Test 1 92 Per Test Stock Available
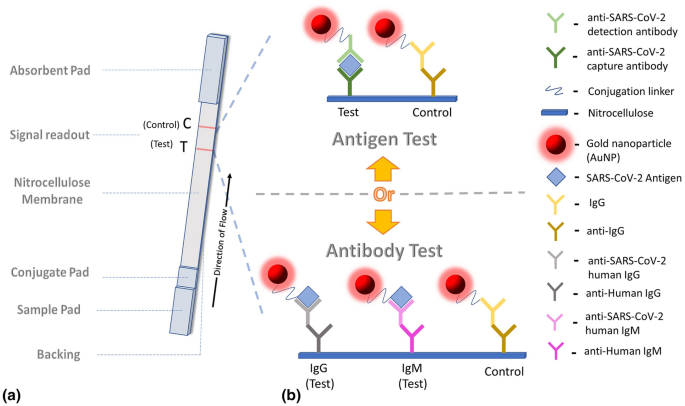
Rapid Testing For Coronavirus Disease 2019 Covid 19 Springerlink